Automation, zBlog
Pharmacovigilance the Significance of Automation in the Manufacturing of Safer Drugs
trantorindia | Updated: April 29, 2021

Life science companies spend millions of dollars on R&D to develop a new drug and seek necessary regulatory approvals to introduce it in the market. Yet, they may need to withdraw it after the launch if it results in Adverse Drug Reactions (ADRs) are risking patient safety and human lives. They may suffer hefty financial losses and face the music from regulatory authorities in this situation. The implications of ADRs are enormous for both public health safety and pharma companies. That’s why the role of Pharmacovigilance (PV) with regards to drug safety in the healthcare sector can’t be understated.
The World Health Organization (WHO) defines PV as “the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems.” It is an indispensable process to reduce the risk of ADRs, improve patient care and ensure regulatory compliance during the drug development as well as post-launch phases on an ongoing basis.
However, Current Challenges in PV Landscape are a Concern
Traditionally, PV processes are data-intensive and resource-exhaustive and demand countless man-hours. They become vulnerable to human errors, poor quality checks, signal detection delays, and information discrepancies which may adversely impact the drug’s outcome or impact indicators. Given that the pharmaceutical industry is poised to reach the market size of $3,206.3 billion by 2030 and will demand more stringent benefit-risk profiling of drugs, it is crucial to overcome existing PV inadequacies. And, that too faster!
The question is how. Well, Automation is the key to transform the PV processes and deliver the much-need efficiency, quality, and speed. Integration of automation technologies such as Artificial Intelligence (AI), Machine Learning (MA), and Robotic Process Automation (RPA) with PV empowers pharma companies to manufacture drugs without compromising either safety or efficacy.
- Automation Benefits in PV Right from case intake to data mining to reporting, automation can streamline workflow and eliminate manual intervention to a significant extent. It can bring standardization and homogeneity in case processing to filter out data noise and improve quality. It can also free up PV professionals to focus on complex, strategic, and high-value tasks such as audit, proactive risk management, trend analysis, and escalations. Automation also brings huge savings of time, resources, and cost.
- Use Cases of Automation in PV While automation has applications across the spectrum of PV operations, let’s take a look at the top three use cases where it is creating a buzz.
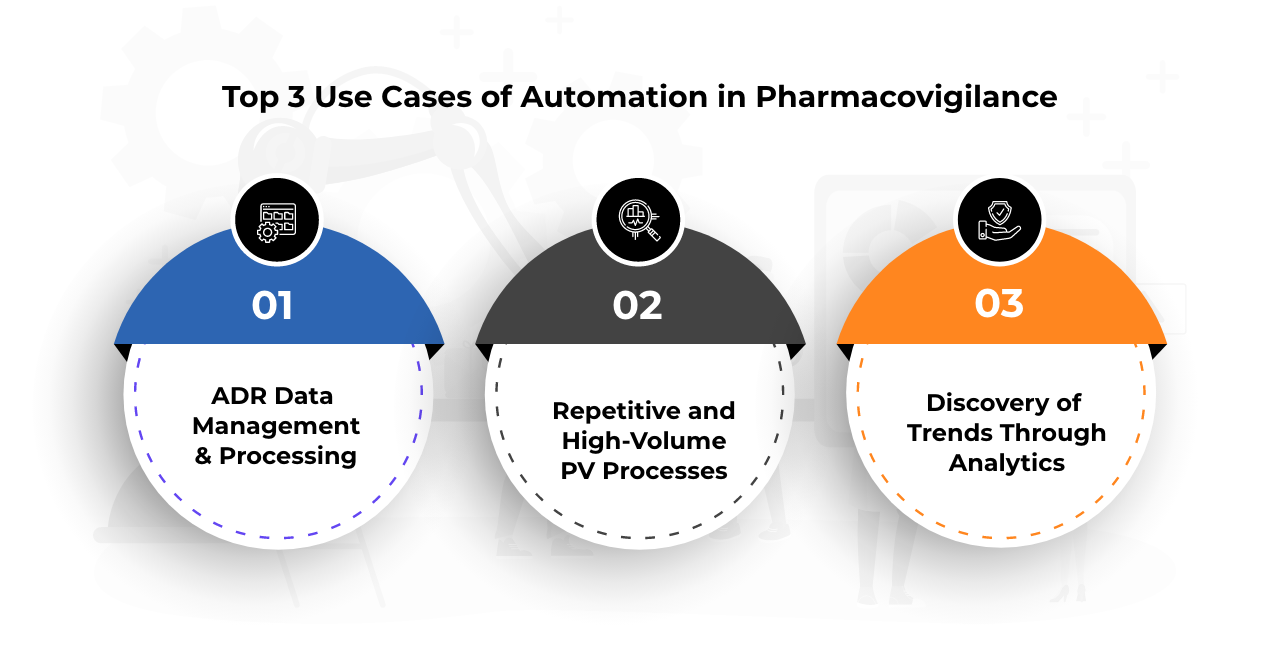
- ADR Data Management and Processing The explosion of big data in healthcare has made it manually impossible to capture and process data at all points. In this scenario, AI can be a game-changer for PV. AI can intelligently and quickly scan through tons of unstructured and semi-structured data from multiple online resources using its advanced search capabilities to capture ADRs. It can leverage Optical Character Recognition (OCR) and Natural Language Processing (NLP) to extract data from pictures, scans, handwritten documents, voice text, or offline records to convert them into machine-readable information. AI can also interpret ADR data to verify its accuracy, duplicity, validity, and priority to segregate them into legitimated cases and false alarms. This can simplify and expedite both the triage process and reporting.
Take for instance the AI-powered PV solution that France is currently using to capture COVID-19 vaccine ADRs in real-time from an online public reporting portal and encode them automatically in MedDRA terminology to rank them by severity. This helps its PV stakeholders to take proactive measures as required.Get in touch with our Automation ExpertContact US - Rule-Based ProcessesRepetitive, high-volume, and error-prone PV processes that follow standard rules are ripe candidates for RPA. Some of the processes that RPA can automate are:
- Data entry and sorting in chronological order
- Preparation of case narratives
- Standardization or coding of data as per international standards
- Quality checks
- Auto-assignment of tasks and alerts to individuals as per their role to reduce manual workarounds
- E-mail acknowledgments and follow-ups
- Daily report generation
- Regulatory documentation and audit filings
- Discovery of Trends and Patterns Through AnalyticsIntegration of AI and ML with predictive analytics acts as a powerful weapon in PV. These technologies can evaluate massive volumes of integrated healthcare data sets to identify meaningful trends and patterns. PV teams can bank upon these real-time insights to generate actionable insights to answer questions such as ‘what will happen’ and ‘what can we do’ with regards to benefit-risk profiling of drugs.
Automation in PV is the need of the hour for the pharma industry. The sooner it embraces automation, the more efficiency it will gain to manufacture safe and high-quality drugs, and reduce ADRs.
Get in touch with Trantor to start your PV automation journey today.